Answer the following:
(i) Haloalkanes easily dissolve in organic solvents, why?
(ii) What is known as a racemic mixture? Give an example.
(iii) Of the two bromo derivatives, C6H5CH(CH3)Br and C6H5CH(C6H5)Br, which one is more reactive in SN1 substitution reaction and why?
(i) Haloalkanes can easily dissolve in organic solvents of low polarity because the new forces of attraction set up between haloalkanes and the solvent molecules are of same strength as the forces of attraction being broken.
(ii) An equimolar mixture of the enantiomers (dextro or laevo forms) is called racemic mixture.
For example: When a 3o halide undergoes substitution with KOH, the reaction proceeds through SN1 mechanism forming racemic mixture in which one of the products has the same configuration as the reactant, while the other product has an inverted configuration.
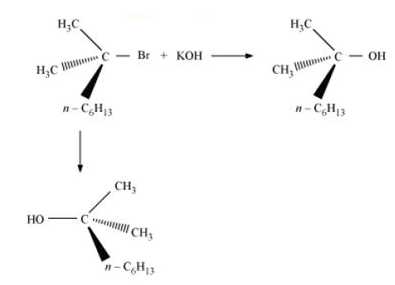
(iii) The SN1 substitution reaction involves the formation of carbocation, which is not affected by the presence of bulky groups.
Thus, C6H5CH(C6H5)Br will be more reactive towards SN1 substitution reaction forming racemic mixture.