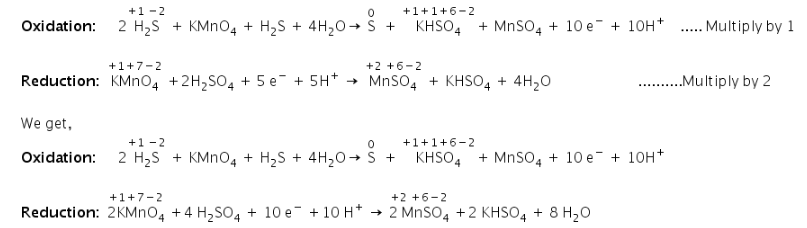H2 S + KMnO4 + H2 SO4 → S+ MnSO4 + KHSO4 + H2 O
Balance by oxidation number method step by step explain please
Step 1
The skeleton equation is:
H2 S + KMnO4 + H2SO4 → S+ MnSO4 + KHSO4 + H2 O
Step 2
a) The oxidation number of various atoms involved in the reaction.
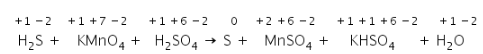
(b) Identify and write out all the redox couple in reaction.
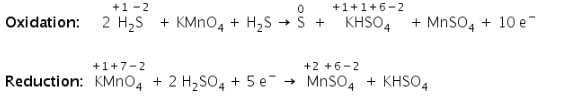
Step 3
Balance the atoms in each half reaction
a) Balance all other atoms except hydrogen an oxygen
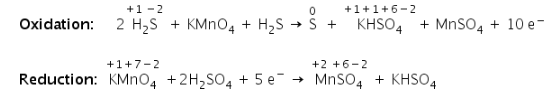
b) Balance the charge:
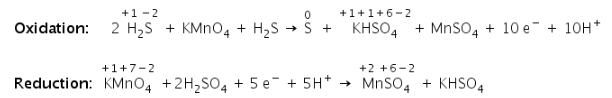
c) Balance the oxygen atoms
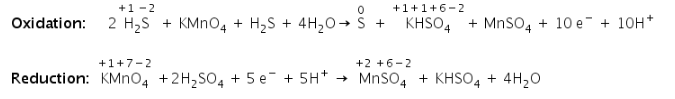
Step 4: Make electron gain equivalent to electron lost.
Step 5: Add the half-reactions together.
Step 6 : Simplify the equation: