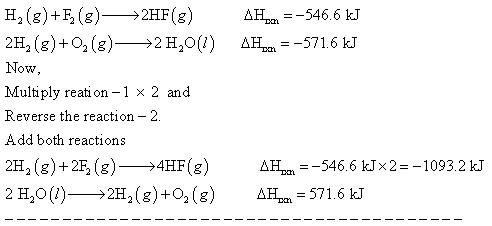H2(g) + F2(g) ------> 2HF (g) Delta H rxn = -546.6 kJ 2H2(g) + O2 (g) -----> 2H2O (l) Delta H rxn = -571.6 kJ Calculate the value of delta H rxn for: 2F2(g) + 2H2O(l) ------> 4HF(g) + O2 (g)
Answer:
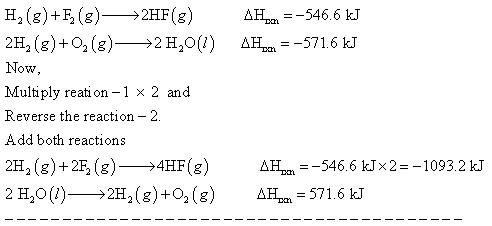
H2(g) + F2(g) ------> 2HF (g) Delta H rxn = -546.6 kJ 2H2(g) + O2 (g) -----> 2H2O (l) Delta H rxn = -571.6 kJ Calculate the value of delta H rxn for: 2F2(g) + 2H2O(l) ------> 4HF(g) + O2 (g)
Answer: