Given the following thermodynamic data,calculate the lattice energy of CaBr2(s).
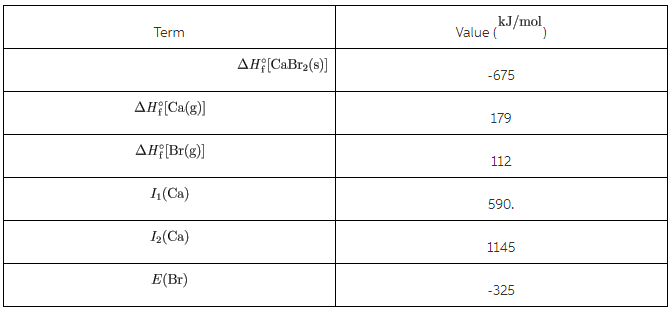
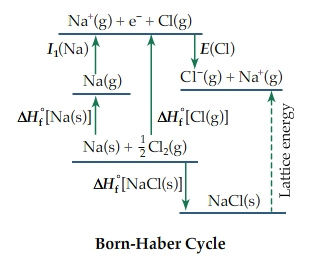
You are also given information on the Born-Harber Cycle which isbelow
Answer:
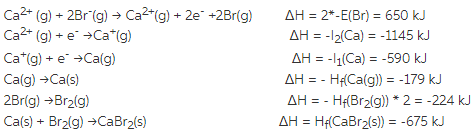
over all eqn Ca2+ (g) + 2Br-(g) →CaBr2(s)
ΔH = 650 -1145-590-179-224-675 = - 2163 kJ/mol