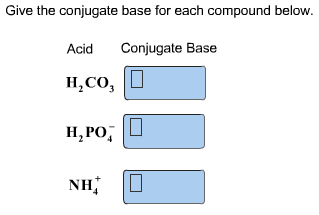
Give the conjugate base for each compound below.
Concepts and reason
The conjugate acid of a base is formed by adding a proton ![]() to the base. Similarly, the conjugate base of an acid is formed by removing a proton from the acid.
to the base. Similarly, the conjugate base of an acid is formed by removing a proton from the acid.
Fundamentals
According to Bronsted-Lowry theory an acid is a species which can donate a proton and base is a species which can accept a proton.
Answer:
Explanation:
Explanation:

Explanation:
As ![]() has +1 charge, by removing one proton, the resultant conjugate base becomes neutral.
has +1 charge, by removing one proton, the resultant conjugate base becomes neutral.



