Give the characteristic tests for the following gases:
![]()
Answer:
The characteristic test for
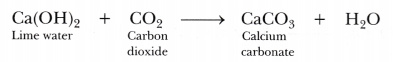
- Carbon dioxide (C02) gas turns lime water milkv when passed through it due to the formation of insoluble calcium carbonate.
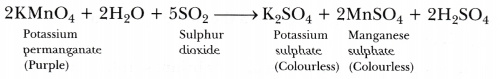
- Sulphur dioxide (S02) gas when passed through acidic potassium permanganate solution (purple in colour) turns it colourless because S02 is a strong reducing agent.
- The evolution of oxygen (02) gas during a reaction can be confirmed by bringing a burning candle near the mouth of the test tube containing the reaction mixture. The intensity of the flame increases because oxygen supports burning.
- Hydrogen (H2) gas burns with a pop sound when a burning candle is brought near it.