Give reasons for the followings:
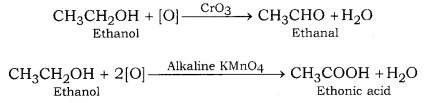
(i) Oxidation of ethanol with CrO3 produces ethanal while ethanol when oxidised with alkaline KMnO4, produces ethanoic acid.
(ii) Propanone forms addition product with HCN.
(iii) Alcohol supplied for industrial purposes is mixed with Copper Sulphate.
(i) When ethanol reacts with chromic anhydride, only partial oxidation occurs and ethanol is formed. On the other hand, when ethanol is heated with alkaline KMn04, it produces ethanoic acid due to complete oxidation.
(ii) When propanone react with hydrogen cyanide, a molecule of H_CN is added across the carbon-oxygen double bond of propanone. Hence, addition reaction occurs.
(iii) To prevent the misuse of alcohol supplied for industrial purposes, it is made unfit for drinking. This can be done by mixing it with poisonous substances such as copper sulphate, methanol, pyridine, etc. The alcohol thus, obtained is called denatured alcohol.