Give reason for the following:
(i) Phenol is more acidic than methanol.
(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (109° 28 ).
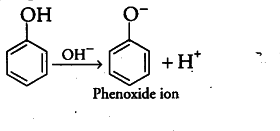
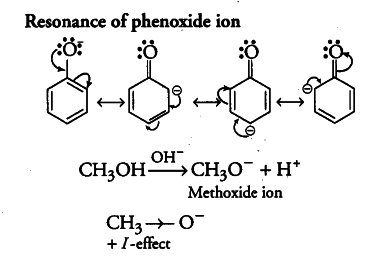
(i) The phenoxide ion, produced by the loss of a proton by phenol, is stabilised by resonance due to delocalisation of the negative charge on the benzene ring. In contrast, methoxide ion, however is not stabilised by resonance. On the other hand, it is further destabilised by positive inductive effect of alkyl group.
(ii) In alcohols, both the oxygen and the carbon attached to it are s{{p}^{3}}-hybridised. Two of the four s{{p}^{3}} -hybridised orbitals of oxygen overlap separately with ls-orbital of H and a s{{p}^{3}} orbital of carbon of the alkyl group to form O—H and C—O, G-bonds respectively, while the remaining two s{{p}^{3}} -orbitals contain lone pairs of electrons. The C—O—-H bond angle is slightly less (108.9°) than the tetrahedral angle (109° 28’) due to greater lone pair-lone pair repulsions than lone pair-bond pair repulsions.