Give balanced chemical equations for the following:
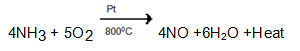
- Catalytic oxidation of ammonia
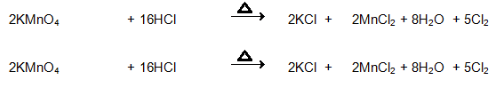
- Conc. Hydrochloric acid reacts with potassium permanganate
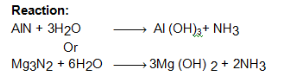
- Hydrolysis of magnesium nitride
Give balanced chemical equations for the following:
Catalytic oxidation of Ammonia
A mixture of dry air and dry ammonia in the ratio of 10:1 by volume is compressed and then passed into a platinum gauze which acts as catalyst at about 800°C.
Conc. Hydrochloric acid reacts with potassium permanganate to give magnesium chloride, potassium chloride and chlorine gas.
Hydrolysis of magnesium nitride
Ammonia gas can be prepared by the action of warm water on nitrides of metals such as magnesium or aluminium.