- Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
- Name the products formed when ethane burns in air. Write the balanced chemical equation for the reaction showing the types of energies liberated.
- Why is reaction between methane and chlorine in the presence of sunlight considered a substitution reaction?
Answer:
- Br2-water test: Br2-water is a brown coloured liquid.
Unsaturated hydrocarbons give addition reaction with Br2, so the colour of Br2-water gets decolourised.
Saturated hydrocarbons do not react with Br2-water, so the colour of Br2-water does not get decolourised. - On burning ethane in air, the products obtained are carbon dioxide and water, along with heat and light.
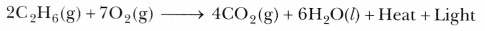
- Methane reacts with chlorine in the presence of sunlight to form chloromethane and hydrogen chloride.

With the excess of chlorine, all the four hydrogen atoms of methane are replaced by chlorine atoms to form carbon tetrachloride (CCl4). This reaction is considered as substitution reaction because hydrogen of methane is substituted by chlorine.