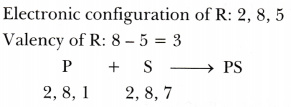Four elements P, Q, R and S belong to the third period of the Modern Periodic Table and have respectively 1, 3, 5 and 7 electrons in their outermost shells. Write the electronic configurations of Q and R and determine their valencies. Write the molecular formula of the compound formed when P and S combine.
Answer:
Electronic configuration of Q : 2, 8, 3
Valency of Q : 3