Concepts and reason
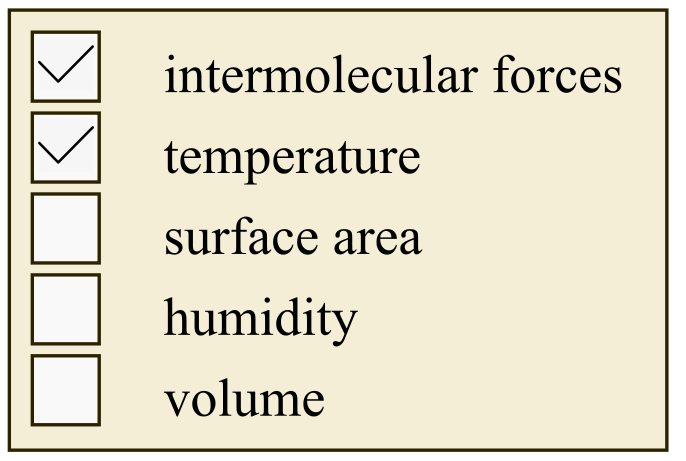
The vapor pressure of a liquid is the pressure exerted by vapors of liquid on the surface of liquid when equilibrium is attained between liquid and its vapor.
Fundamentals
There are some properties which affect the vapor pressure of liquid such as temperature and intermolecular forces.
Answer:
Vapor pressure is independent of humidity.
For a certain amount of water vapor in the air, the property that determines the vapor pressure is only temperature. Humidity will affect the vapor pressure only when all other variables are fixed.
Do not confuse between temperature and humidity.
Vapor pressure is independent of volume.
The liquid in a container is in equilibrium with its vapor. When volume is increases, some of the liquid molecules changes into vapor phase. When volume is decreases, some of the vapor changes into liquid phase.
Intermolecular forces are the forces that hold two or more atoms in a molecule. If the intermolecular forces are strong then vapor pressure is relatively low and if the intermolecular forces are weak then vapor pressure is high.
If the intermolecular forces are strong then molecules will not easily leave the liquid surface resulting in low vapor pressure.
As the temperature increases, vapor pressure of the liquid increases.
As the temperature increases, kinetic energy of the molecules increases. With increasing kinetic energy intermolecular forces decreases. Due to this, molecules can easily escape from surface of liquid. Therefore, vapor pressure increases.
Vapor pressure is independent of surface area.