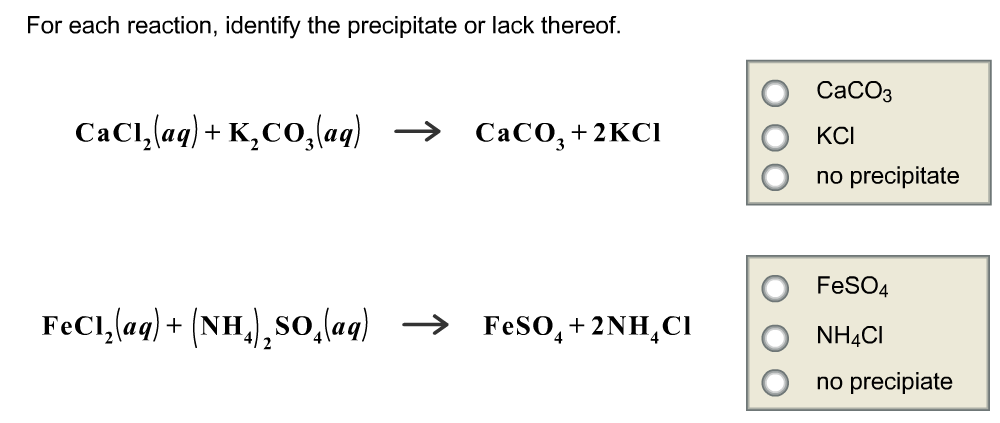For each reaction, identify the precipitate or lack there of.
Answer:
According to solubility rules, precipitate is the one which is insoluble in water.
CaCl2 + K2CO3 ---- > CaCO3 + KCl
CaCO3 is insoluble in water and it is a precipitate
FeCl2 + (NH4)2SO4 ----- > FeSo4 + 2NH4Cl
In tis reaction no precipitate is formed.