For each metal complex, give the coordination number for the metal species.
Concepts and reason
Metal complexes are coordination compounds that retain their properties when dissolved in water. All metal complexes consist of the central metal and ligands.
Fundamentals
Coordination Number (CN):
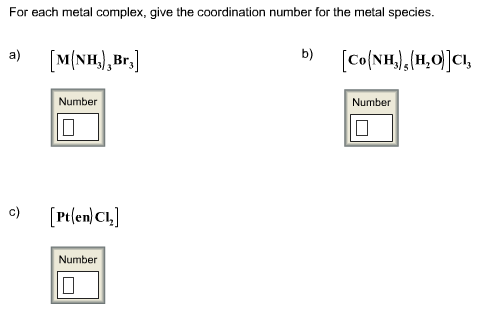
The coordination number is defined as the total number of dative bonds or coordinate bonds that are linked between a central metal atom or ion and ligands.
Consider a metal complex as shown below:
Answer:
(a)
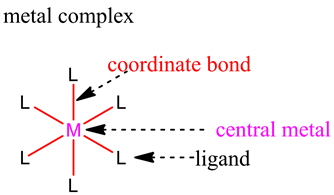
The structure of the metal complex is as follows:
The coordination number = 6.

(b)
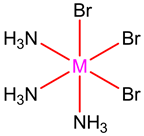
The structure of the metal complex is as follows:
The coordination number = 6.

( c)
The structure of the metal complex is as follows:

The coordination number = 4.
