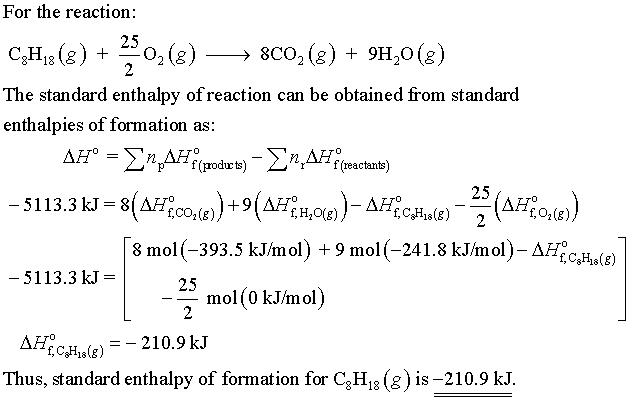
For a particular isomer of C8H18, the following reaction produces 5113.3 kJ of heat per mole of C8H18(g) consumed, under standard conditions.
C8H18 + 25/2(O2) -> 8CO2 + 9H2O
DeltaH= -5113.3 kJ
What is the standard enthalpy of formation of this isomer of C8H18(g)?
Answer: