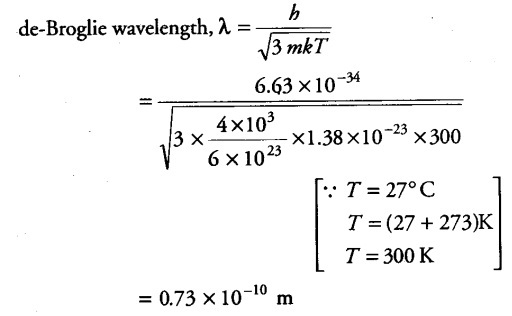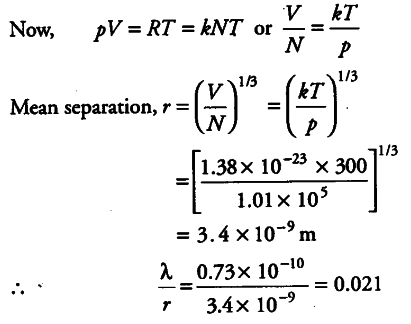Find the typical de-Broglie wavelength associated with a He atom in helium gas at room temperature (27° C) and 1 atm pressure and compare it with the mean separation between two atoms under these conditions.
Mass of helium atom, m = Atomic height / Avogadro’s number = 4 x {{10}^{3}} / 6 x {{10}^{23}} g
Boltzmann’s constant , k = 1.38 x {{10}^{-23}} J mol/K