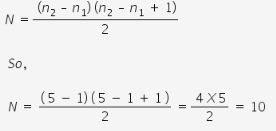Find the number of spectral lines obtained when electron de-excites from 5th to the 1st energy level but no line is seen in Balmer series?
If the electron jumps from n2 = 5 to n1 = 1 then following are the transitions possible.
- 5→ 4
- 5→ 3
- 5→ 2
- 5 → 1
- 4 → 3
- 4→ 2
- 4→ 1
- 3 → 2
- 3→ 1
- 2 → 1
Hence there are 10 transitions and hence 10 spectral lines possible.
The general formula for the number of spectral lines emitted is