Find the difference in the oxidation numbers present of the two types of sulphur present in Na2S4O6 [sodium - 2 sulphur - 4 oxygen - 6].
(Hint :- sulphur-sulphur linkages present)
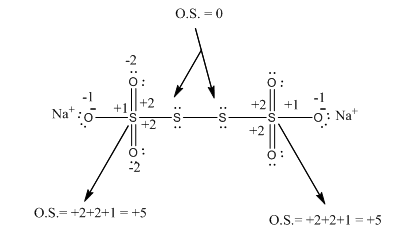
In the case of Na2S4O6, if we observe the structure of this compound it is found that there are two types of sulphur atoms
- O.S. of first sulphur: sulphur is attached to the same sulphur atoms hence there is no change in the electronic arrangement of this sulphur atom, therefore, its O.S. is zero.
- O.S. of second sulphur: This atom of sulphur is attached to the three oxygen atoms two are doubly bonded and one is singly bonded
Therefore for doubly bonded oxygen will exert the charge +2 and for singly bonded oxygen will +1. hence on total, the O.S. of Second sulphur is +5.