- Chlorine occurs in nature in two isotopic forms with masses 35 u and 37 u. The percentage of occurrence of 35Cl is 75%. Find the average atomic mass of chlorine atom.
- Give any three applications of isotopes.
- Percentage of
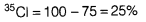
Average atomic mass =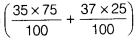 =
= 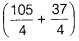 =
= 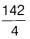 = 35.5
= 35.5 - Applications of isotopes
- An isotope of iodine (1-131) is used in the treatment of goitre.
- Phosphorus (P-32) is used in agricultural research.
- Sodium (Na-24) is used to detect blood clots and tumours.