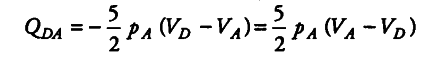A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. Find heat exchanged by the engine with the surroundings for each section of the cycle. [{ C }_{ v } = (3/2)R]
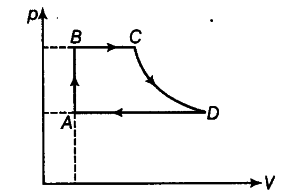
(i) AB: constant volume
(ii) BC: constant pressure
(iii) CD: adiabatic
(iv) DA: constant pressure
(i) In figure, portion AB of the cycle involved increases in pressure/temperature of gas at constant volume. Therefore, the system gains heat from the surroundings.
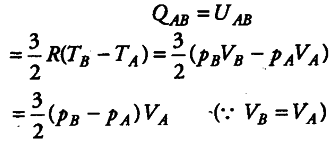
(ii) In the portion BC, the gas increases in volume at constant pressure. Heat required for this is gained from surroundings.
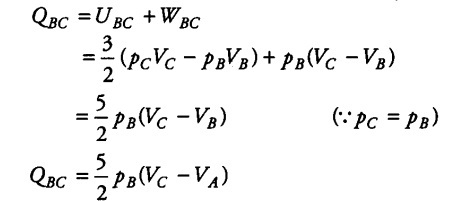
(iii) As CD represents an adiabatic change, therefore, { Q }_{ CD } = 0.
(iv) DA involves compression of gas from { V }_{ D } to
{ V }_{ A } at constant pressure
{ p }_{ A }. Work is done on the gas.