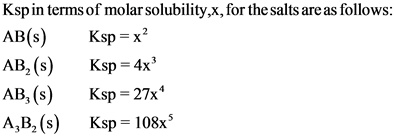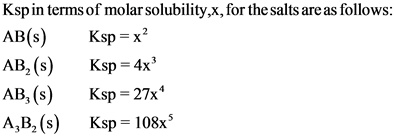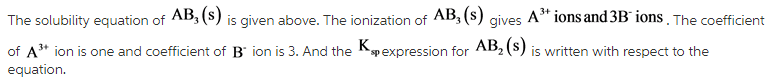Express Ksp in terms of molar solubility, x, for a salt with a formula of
1. AB(s)
2. AB2(s)
3. AB3(s)
4. A3B2(s)
Concepts and reason
Solubility:
Solubility is defined as the maximum amount of solute dissolved in a given amount of solvent to make a saturated solution at a particular temperature.
Molar solubility:
The molar solubility is the number of moles of solute that dissolves in a litre of solution. It is expressed as mol/L or M (molarity).
Fundamentals
Solubility product: The equilibrium constant which defines the solubility of ionic substance in water is known as solubility product. The solubility product is denoted by![]() .
.
For a saturated solution, the solubility product is the product of activities of the ions, each raised to its coefficients (powers).
Consider a general reaction:
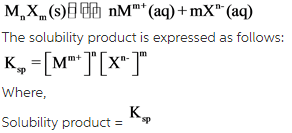
The molar solubility of M ion = [M]
The molar solubility of X ion = [X]
The ions raised to the power= n and m
Answer:
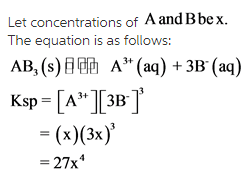
Let concentrations of A and B be X
The equation is as follows:

The solubility equation of AB(S) is given above. The ionization of AB(S) gives ![]() .
.
The coefficient of ![]() ion is one and coefficient of
ion is one and coefficient of ![]() ion is one. And the
ion is one. And the ![]() expression for AB(S) is written with respect to the equation.
expression for AB(S) is written with respect to the equation.