Explain with suitable diagram, two different processes used for separating -; a mixture of two liquids both miscible and immiscible.
Method of separation of two miscible liquids:
Fractional distillation (To separate the mixture of miscible liquid X having lower boiling point and miscible liquid Y with higher boiling point )
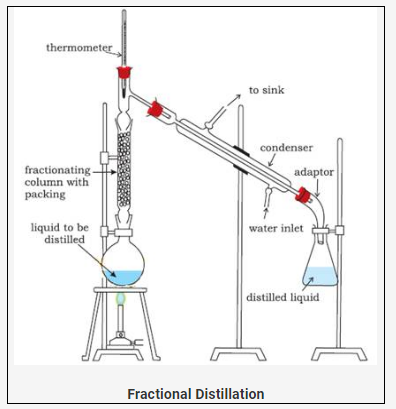
Technique
· Mixture X + Y is kept in a distillation flask A attached with a fractionating column having glass beads.
· The flask is then carefully heated.
· The mixture first evaporates and later gets condensed. The glass beads present in the fractional column provides larger surface area for the vapours to cool down.
· This technique is used to separate the mixtures having their temperature difference less than 30oC.
Separation of Compounds
· Liquid with the higher boiling point Y remains in the flask A after condensation.
· Liquid with the lower boiling point X gets collected in the flask B after condensation.
Example: Benzene + Toluene;
Water + Carbon tetrachloride
Method of separation of two immiscible liquids:
Separating funnel (To separate the mixture of immiscible heavier liquid X from immiscible lighter liquid Y)
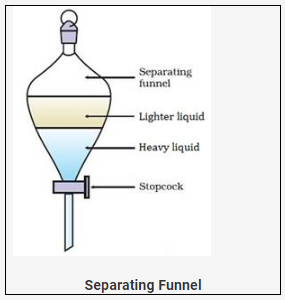
Technique
· Take a separating funnel.
· Pour mixture of X + Y from the top of a separating funnel.
· Allow the mixture to stand in the funnel for sometime till both the layers separate out.
· The two distinct layers are formed. (Heavier-below and lighter-above)
Separation of Compounds
· Heavier liquid X can be collected first, in a beaker on opening the tap/stop cork.
· Lighter liquid X remains in the separating funnel which can be collected in another beaker.
Example:
Water + Oil; Kerosene + Oil