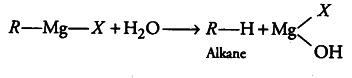Explain
(i) why the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) why alkyl halides, though polar, are immiscible with water?
Or
why is the solubility of haloalkanes in water very low?
(iii) why Grignard reagents should be prepared under anhydrous conditions?’
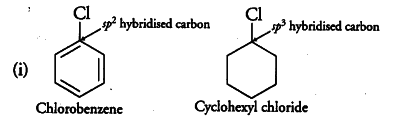
Due to {{sp}^{2}} hybridisation of C-atom in chlorobenzene, C-atom is more electronegative
(greater s-character) whereas in cyclohexyl chloride,C-atom is sp hybridised, i.e. less electronegative
(lesser s-character). So, polarity of C—Cl bond in chlorobenzene is less than the C—Cl bond in cyclohexyl chloride. Further, due to delocalisation of lone pair of electrons of Cl-atom over the benzene ring, C—Cl bond in chlorobenzene acquires some double bond character while C—Cl bond in cyclohexyl chloride is a pure single bond. Thus, C—Cl bond in chlorobenzene is shorter than in cyclohexyl chloride. As dipole moment is a product of charge and distance, therefore, the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
(ii) Water molecules have enough strong intermolecular H-bonding which is difficult to be broken by alkyl halides, though they are polar in nature as well. Therefore, alkyl halides do not dissolve in water and form separate layers.
(iii) Grignard reagents (R—Mg—X) are readily decomposed by water to produce alkanes. That is why, they should be prepared under anhydrous conditions. Instead, ether is used as a solvent during the preparation of Grignard reagent.