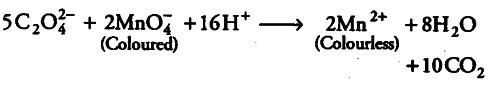When oxalic acid is added to acidic solution of KMnO _{ 4 }, its colour disappear due to the reduction of MnO _{ 4 } ion to {{Mn}^{2+}}. Chemical reaction occurring during this neutralisation reaction is as follows:
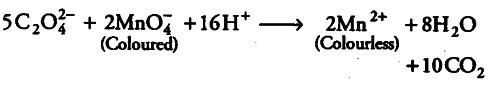
When oxalic acid is added to acidic solution of KMnO _{ 4 }, its colour disappear due to the reduction of MnO _{ 4 } ion to {{Mn}^{2+}}. Chemical reaction occurring during this neutralisation reaction is as follows: