Definition : It is defined as the electrostatic force of attraction which exist between the covalently bonded hydrogen atom of one molecule and the electronegative atom of other molecule
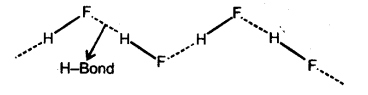
Cause : An electrostatic force of attraction between hydrogen atom of one molecule and electronega-tive atom of 2nd molecule. XA
Conditions : (1) Hydrogen atom should be con-nected to highly electronegative atom say F, O or
N.
(2) The electronegative atoms should be small in size. A
Strength : It is a very weak bond, weaker than ionic, covalent bond.
Types of H-Bonding : There are two types of hydrogen-bonds.
(i) Intermolecular hydrogen bond : If is formed
between two different molecules of the same or different compounds. For example H-bond in case of HF, alcohol and water.
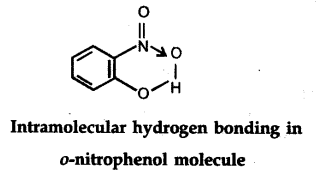
(ii) Intramolecular hydrogen bond : It is formed when hydrogen atom is in between the two highly electronegative (F, O, N) atoms present within the same molecule. For example, in o-nitrophenol the hydrogen is in between the two oxygen atoms