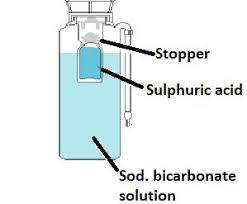
Explain the working of soda acid type fire extinguisher
Soda acid type extinguisher acts by cutting off supply of air. It acts on all types of fire except due to electrical and inflammable liquids. The carbon dioxide is liberated by the action of acid on baking soda. It increases the percentage of carbon dioxide in air (CO2 is non supporter of combustion).
This type of fire extinguisher contains a bottle of sulphuric acid supported by a metallic container filled with a baking soda solution.When the cylinder is inverted and knob struck, against the ground, the acid bottle breaks and the acid comes into contact with the backing soda.
2NaHCO3 + H2SO4-> Na2SO4 + 2H2O + 2CO2
Baking sulphuric sodium water carbon
soda acid sulphate dioxide
As a result carbon dioxide is liberated. This increases the percentage of carbon dioxide in air. Due to this the supply of air is cut off and there, fire is extinguished. These types of extinguishers are used in cinema halls, multistorey buildings, etc