In a chemical reaction, sometimes, an excess of one or more substance is available.
Naturally, some of these excess substances will be left over when the reaction is complete.
The reaction stops immediately as soon as one of :the reactant is totally consumed.
Consider a chemical reaction given below initiated by passing a spark through a reaction vessel containing 16 moles of {{H}_{2}} and 10 moles of {{O}_{2}}.
The balanced equation could be
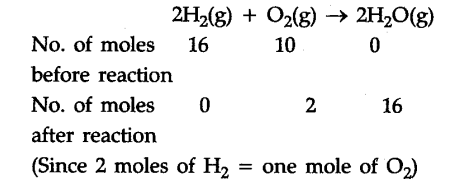
From the above example it is clear that the reaction stops after the consumption of 8 moles of {{O}_{2}} since no further amount of {{H}_{2}} is left to react with unreacted {{O}_{2}}
The substance that is completely consumed is called limiting reagent as it limits the amount of the product formed.
The other substance present in excess are called excess reagent. Here {{O}_{2}} is excess reagent