Explain the term ‘Metallurgy’. What are the steps involved in the extraction of metals from their ores? Explain in brief each process with relevant examples.
Metallurgy
The scientific principles and the physical and chemical processes that are applied to obtain pure metals from their ores are known as metallurgy.
In other words, the process used for the extraction of metals in their pure form from their ores is referred to as metallurgy.
The extraction of a metal from its ore depends on:
- The type of ore being used.
- The nature of the impurities present in the ore.
- The degree of the reactivity of the metal that is to be extracted.
Steps involved in Extraction
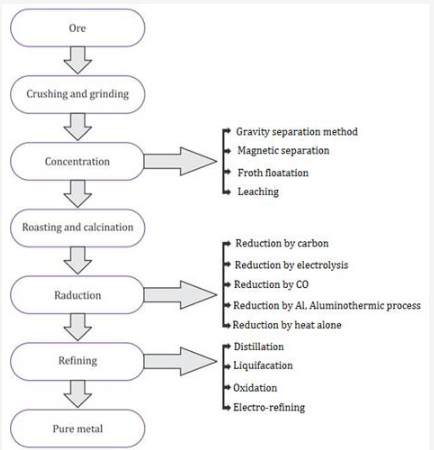
rushing and Grinding:
Ores are crushed into a fine powder in big jaw crushers and ball mills. This process is called pulverization .
A. CONCENTRATION OF ORES (Ore Dressing)
The process of removing gangue (earthly impurities) from an ore is known as concentration or dressing of ore.
I. Gravity separation (Hydraulic washing) :
Principle: Separation of Ore and Gangue.
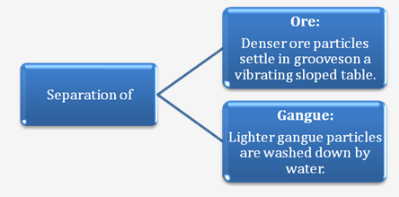
Process:
The ore is poured over a vibrating sloped table with grooves and a jet of water is allowed to flow over it. The dense ore particles settle down in the grooves.
II. Magnetic Separation:
Principle : Separation of Magnetic ore and Non-magnetic ore.
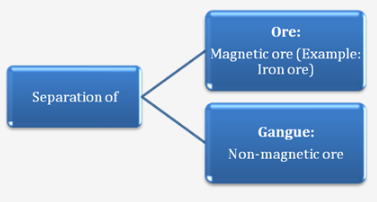
Process:
B. The pulverized ore is placed on a conveyor belt.
C. The magnetic particles are attracted to the magnetic wheel and fall separately apart from the non-magnetic particles.
III. Froth Flotation
Principle: Separation of ore and gangue by wetting with oil and water.
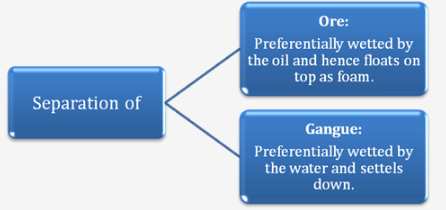
Process:
i. The method is generally applied for sulphide ores.
ii. The ore is taken in a large tank containing oil and water and is agitated with a current of compressed air. The ore is wetted by the oil and separates from the gangue in the form of froth.
D. CONVERSION OF THE CONCENTRATED ORE INTO METALLIC OXIDE
Concentrated ore is converted into metal oxide either by Roasting or Calcination .
I. Roasting
Process of heating the concentrated ore to a high temperature in the presence of excess air.
Examples:*
![]()
![]()
II. Calcination:
Process of heating the concentrated ore in the absence of air at a temperature not sufficient to melt the ore.
![]()
![]()
E. REDUCTION OF METALLIC OXIDES TO METAL (SMELTING)
The process of removing oxygen from a metallic oxide so as to convert it (metallic oxide) into a metal is known as reduction or smelting .
I. Reduction by Electrolysis
Reduction of highly electropositive (reactive) metals like K, Na, Ca, Mg, Al oxides/halides.
Electrolysis of fused metallic salts
Example: Extraction of Aluminium [Al2O3.2H2O]
When electric current is passed through the concentrated ore, the reaction takes place as follows:
![]()
At Cathode: 2Al+3 + 6e- → 2Al
At Anode : 3O2- - 6e- → 3[O] → 3O2 (gas)
Product at cathode: Pure aluminium metal
Product at anode: Oxygen gas
II. Reduction by Reducing agents
In this process the metallic oxide is reduced to metal by using reducing agents like carbon (coke, charcoal), carbon monoxide. Hydrogen etc.
Example: Reduction of Zn, Fe, Pb, Cu oxides.
![]()
![]()
![]()
![]()
III. Reduction by thermal decomposition
Oxides of metals like mercury and silver get reduced to their corresponding metals on heating above 300oC. They do not require reducing agent or electrolytic reduction for this purpose.
![]()
F. REFINING OF IMPURE METAL
- Distillation refining : For refining volatile metals e.g. zinc, mercury.
- Liquation : For refining low melting point metals e.g. lead, tin.
- Oxidation refining : For refining metals by oxidation of their impurities e.g. iron.
- Electrolytic refining : For refining impure metals by electrolysis e.g. Cu, Al, Pb.