Explain the shape of BCl3 using VSEPR theory.
Ground state configuration of B: 1s2 2s2 2p1.
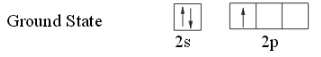
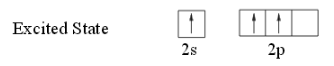
Since the formation of three bonds with chlorine atoms require three unpaired electrons, there is promotion of one of 2s electron into the 2p sublevel by absorbing energy.
So the configuration becomes 1s2 2s1 2px12py1.
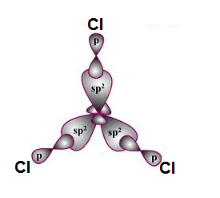
Boron undergoes sp2 hybridization by using a 2s and two 2p orbitals to give three half filled sp2 hybrid orbitals which are oriented in trigonal planar symmetry.
![]()
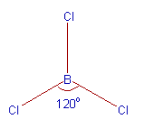
Boron forms three σsp-p bonds with three chlorine atoms by using its half filled sp2 hybrid orbitals. Each chlorine atom uses it’s half filled p-orbital for the σ-bond formation. Thus the shape of BCl3 is trigonal planar with bond angles equal to 120o.