Explain the important aspects of resonance with reference to the ${ CO }_{ 3}^{2 - }$ ion.
When a single Lewis structure of a molecule cannot describe its all properties, a number of structures, called canonical structures, are written.
The actual structure, known as resonance hybrid, is in between of all these canonical forms. This phenomenon is called resonance. According to experimental findings, all carbon to oxygen bonds in { CO }_{ 3 }^{ 2- } are equivalent, while in a single Lewis structure, there are two single bonds and one double bond between carbon and oxygen. So, a single Lewis structure is inadequate for the representation of
{ CO }_{ 3 }^{ 2- } ion. The carbonate ions is best described as a hybrid of the canonical or resonance forms I, II and III.
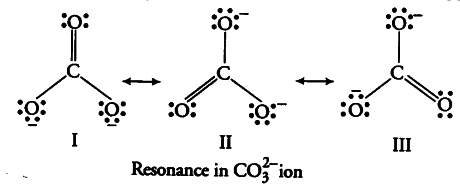
All canonical forms have similar energy, same positions of atoms and same number of bonded and non-bonded pairs of electrons. Thus, in { CO }_{ 3 }^{ 2- } all the C—O bonds are equivalent.