Explain the formation of the following molecules using valence bond theory.
a) $N _{ 2 }$ molecule b) $O _{ 2 }$ molecule
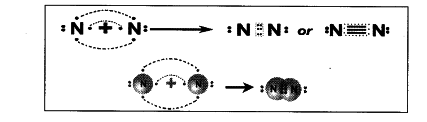
a) Formation of N _{ 2 } molecules by valence bond theory :
- The electronic configuration of ‘N’ atom is 2, 5 and to have Octet in the valence shell it requires three more electrons.
- When two nitrogen atoms approach each other, each atom contributes 3 electrons for bonding.
- There are six electrons shared between two nitrogen atoms in the form of three pairs.
- Therefore, there is a triple bond between two nitrogen atoms in N _{ 2 } molecules.
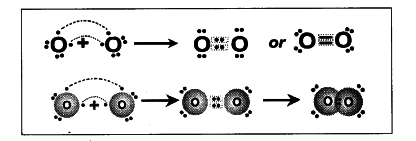
b) Formation of O _{ 2 } molecule by valence bond theory :
- The electronic configuration of { }_{ 8 }{ O } is 2, 6.
- Oxygen atom has six electrons in its valence shell.
- It requires two more electrons to get Octet in its valence shell.
- Therefore oxygen atoms come close and each oxygen atom contributes two electrons for bonding.
- Thus there exist two covalent bonds between two oxygen atoms in O _{ 2 } molecule as there are two pairs of electrons shared between them.
- Two pairs of electrons are distributed between two oxygen atoms.
- So, we can say that a double bond is formed between two oxygen atoms in O _{ 2 } molecule.
- By viewing the following diagram, both the oxygen atoms have Octet in the valence shell.