Explain the following reactions. .
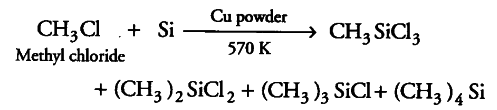
(i) Silicon is heated with methyl chloride at high temperature in the presence of copper.
(ii) Silicon dioxide is treated with hydrogen fluoride.
(iii) CO is heated with ZnO.
(i) When Si is heated with CH _{ 3 }Cl at high temperature in the presence of Cu as a catalyst, a mixture of mono-, di- and trimethylchlorosilanes along with a small amount of tetramethylsilane is formed.
(ii) When Si{{O}_{2}} reacts with HF, silicon tetrafluoride is formed which dissolves in HF to from hydrofluorosilicic acid.
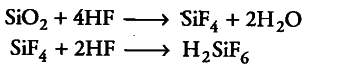
(iii) CO is a strong reducing agent but it cannot reduce ZnO as for CO—> {{CO}_{2}}
${{∆}_{r}}$G° is always higher than that of ZnO. Thus, no reaction takes place.