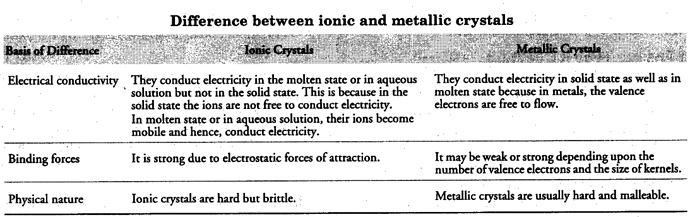
Explain
(i) the basis of similarities and differences between metallic and ionic crystals.
(ii) ionic solids are hard and brittle.
(i) Similarities between ionic and metallic crystals
(a) Both ionic and metallic crystals have electrostatic forces
of attraction. In ionic crystals, these are between the oppositely charged ions. In metals, these are between kernels and valence electrons. That is why, both have high melting points.
(b) In both cases, the bond is non-directional.
(ii) Strong electrostatic forces of attraction among oppositely charged ions make ionic crystals hard and brittle.