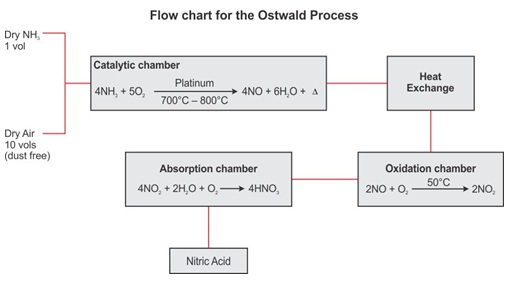
Explain Ostwald process of manufacturing nitric acid.
In 1914, a German chemist Ostwald developed the Ostwald process to manufacture nitric acid.
Step 1: Catalytic oxidation of Ammonia
A mixture of dry air and dry ammonia in the ratio of 10:1 by volume is compressed and then passed into a platinum gauze which acts as catalyst at about 800°C.
4NH3 + 5O2 → 4NO +6H2O +Heat
Step 2: Oxidation of nitric oxide
Nitric oxide combines with oxygen to form nitrogen dioxide at about 50°C.
2NO +O2→ 2NO2
Step 3: Absorption of nitrogen dioxide in water
The nitrogen dioxide and oxygen present in the air react with water to form nitric acid.
4NO2 +2H2O +O2 → 4HNO3
Nitric acid obtained is concentrated above 50%. On further distillation, 68% nitric acid is produced.