Explain isotopes and isobars with the help of suitable examples.
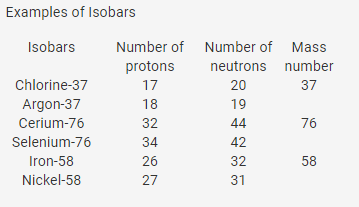
Isotopes
- Atoms of the same element differing in the number of neutrons in their nuclei are known as isotopes. Thus, isotopes of an element have the same atomic number but different atomic mass numbers.
- Isotopes are identified by their mass numbers.
- For example, the isotopes of carbon are referred to as carbon-12, carbon-13 and carbon-14.
- Isotopes of an element have similar chemical properties but different physical properties.
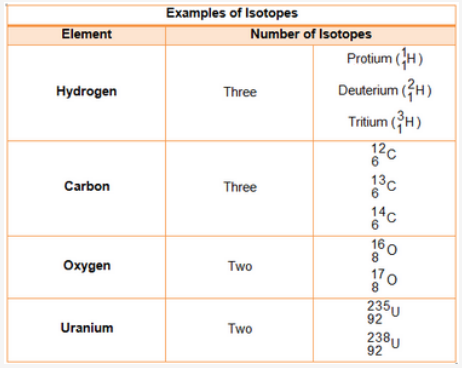
Isobars
The atoms of different elements having different atomic numbers but the same mass number are known as isobars.
Examples of Isobars