Explain how does —OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Phenol can be regarded as resonating hybrid of the following structures:
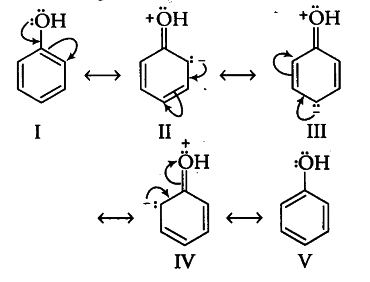
Due to + R effect of the —OH group, the electron density in the benzene ring increases thereby facilitating the attack of an electrophile. In other words, presence of—OH group activates the benzene ring towards electrophilic substitution reactions. Now, since the electron density is relatively higher of the two o- and one p-position, electrophilic substitution occurs mainly at o- and p-positions.