Enter the net ionic equation, including phases, for the reaction of AgNO3(aq) with K2SO4(aq).
How do you solve this? And, because the products of this reaction aren’t given, how do we know which elements pair together in the product?
Concepts and reason
A net ionic equation is a chemical equation in which it indicates only the participating species in a chemical reaction. These net ionic equations are most commonly used in redox reactions, double – displacement reactions and the reaction of strong electrolytes in water.
Fundamentals
There are three steps to write a net ionic equation:
In the first step, the chemical equation should be balanced.
Write the ionic equation by splitting the strong electrolytes into ions in the form of an aqueous solution. The charge and coefficient of each ion should be indicated. Make sure that the (aq) phase should be indicated after writing each ion.
In the net ionic equation, the phase with (s), (l) and (g) cannot be changed. The (aq) phase in both reactants and products cancel each other because they are spectator ions. These spectator ions cannot participate in the reaction.
Example for net ionic equation of 1M of ![]()
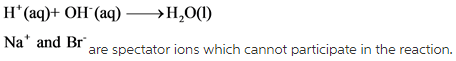
Answer:
The balanced chemical reaction:
![]()
The ionic equation:
![]()
he ionic equation:
![]()
The net ionic equation:
![]()
