Elements from A to F have the distribution of electrons, protons and neutrons in the following way.
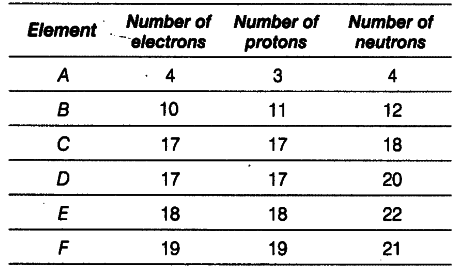
From the table, find
(i) a pair of ionic gas
(ii) an atom of noble configuration
(iii) a pair of isobars
(iv) a pair of isotopes
(i) A and B, because number of electrons and protons are different for these elements.
(ii) E, because it has completely filled outer shell electronic configuration 2, 8, 8(K,L,M)
(iii) E and F, because these have same mass number (number of protons + number of neutrons) but different atomic number.
(iv) C and D, because these have same atomic number (number of protons) but different mass number.