a. Draw the structures of the following:
i. H2S2O8
ii. HClO4
b. How would you account for the following:
i. NH3 is a stronger base than PH3.
ii. Sulphur has a greater tendency for catenation than oxygen.
iii. F2 is a stronger oxidising agent than Cl2.
a)
(i) 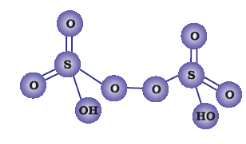
Peroxodisulphuric acid (H2S2O8)
(ii) 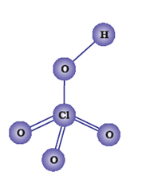
Perchloric acid( HClO4)
b)
(i) Due to smaller atomic size the density of lone pair electrons on N in NH3 is larger than that of P in PH3. So, NH3 is a stronger Lewis base than that of PH3.
(ii) Because of stronger S-S bonds as compared to O-O bonds, sulphur has a greater tendency for catenation than oxygen.
(iii) It is due to:
- low enthalpy of dissociation of F-F bond
- high hydration enthalpy of F-

