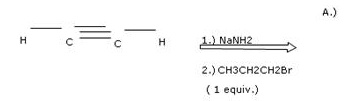
Draw the structures of organic compounds A and B. Indicate stereochemistry where applicable.
Concepts and reason
This problem is based on the concept of alkynes.
Alkynes are the class of organic compounds containing carbon-carbon triple bonds. Alkyne is converted into higher alkyne on reaction with haloalkane in presence of a base. Reduction of alkyne to alkene can be done in the presence of Lindlar’s catalyst.
Fundamentals
Alkynes that have a hydrogen attached to triple bonded carbon are termed as terminal alkynes. The other alkynes are referred as nonterminal alkynes or internal alkynes. Non-terminal alkynes are more stable and have more boiling point that the corresponding terminal alkynes.
Answer:
(1)
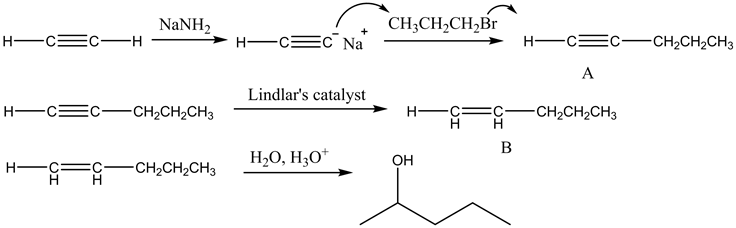
The reaction of acetylene with propyl bromide in presence of sodamide is written as follows:
Part 1
The structure of organic compound A is as follows:
![]()
Ethyne which is a terminal alkyne reacts with sodamide to give acetylides which on reaction with haloalkane produce higher alkyne which is pent-1-yne.
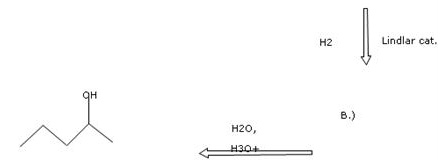
(2)
The reaction of pent-1-yne with Lindlar’s catalyst is as follows:
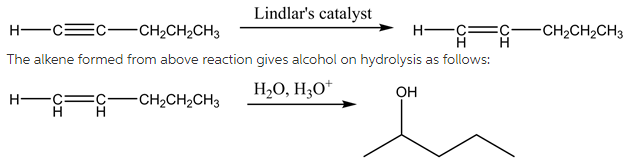
Part 2
The structure of organic compound B is as follows:
![]()
The hydrogenation of alkynes can be easily carried out using deactivated catalysts such as Lindlar’s catalyst to produce cis alkene. The Lindlar’s catalyst is ![]() in lead acetate poisoned with a small amount of quinoline. The acid catalysed reaction of water with alkenes results in the formation of alcohol. The alcohol formed here is 2-pentanol. The addition of water molecule takes place according to Markonikov’s rule which states that highly substituted alkenes are predominantly formed.
in lead acetate poisoned with a small amount of quinoline. The acid catalysed reaction of water with alkenes results in the formation of alcohol. The alcohol formed here is 2-pentanol. The addition of water molecule takes place according to Markonikov’s rule which states that highly substituted alkenes are predominantly formed.
Part 1
The structure of organic compound A is as follows:
![]()
Part 2
The structure of organic compound B is as follows:
![]()