(a) Draw the structures for the following compounds
(i) ethanoic acid
(ii) butanone, C2H5COCH3.
(b) Conversion of ethanol to ethanoic acid is considered an oxidation reaction. Why?
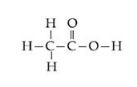
(a)(i) Ethanoic acid [CH3COOH]
(ii) Butanone [C2H5COCH3]
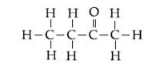
(b) Ethanoic acid has one oxygen atom more and two hydrogen atoms less than ethanol so, the conversion of ethanol to ethanoic acid is an oxidation reaction in which oxygen is added and hydrogen is removed.