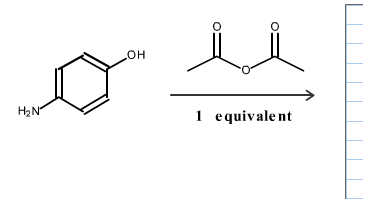
Draw the structure of the aromatic product from the following reaction.
Concepts and reason
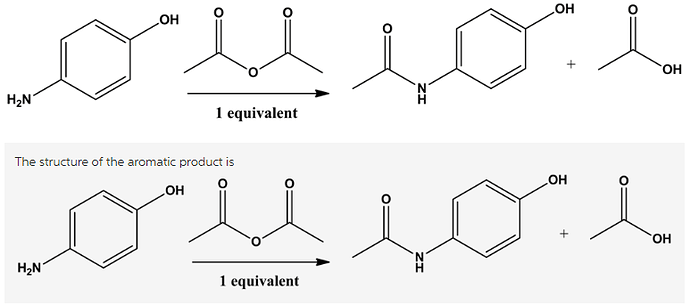
An acid anhydride reacts with amines to form an amide bond. Amide bond consists of CONHgroup, in which NH group acts as a nucleophile and CO group acts as an electrophile.
Fundamentals
Acid anhydrides are the compounds which are produced by removing water molecules from the acid and these acts as nucleophiles.
When an acid anhydride reacts with an amine, it forms a peptide bond.
Answer:
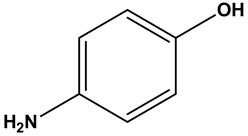
The given substrate is shown below.
Explanation:
The reaction can be written as follows.
Explanation:
The amine group is more nucleophilic than (OH) group. Hydrogen can be removed from amine ![]() group thereby electrophilic attack of anhydride takes place resulting in the formation of peptide bond.
group thereby electrophilic attack of anhydride takes place resulting in the formation of peptide bond.