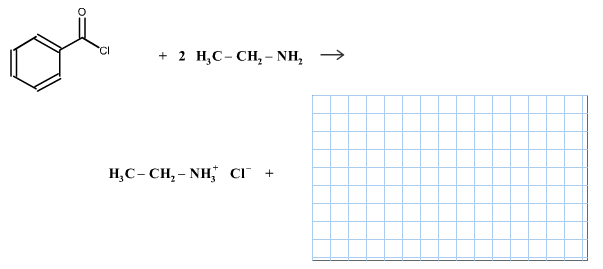
Draw the remaining product of the following reaction.
Concepts and reason
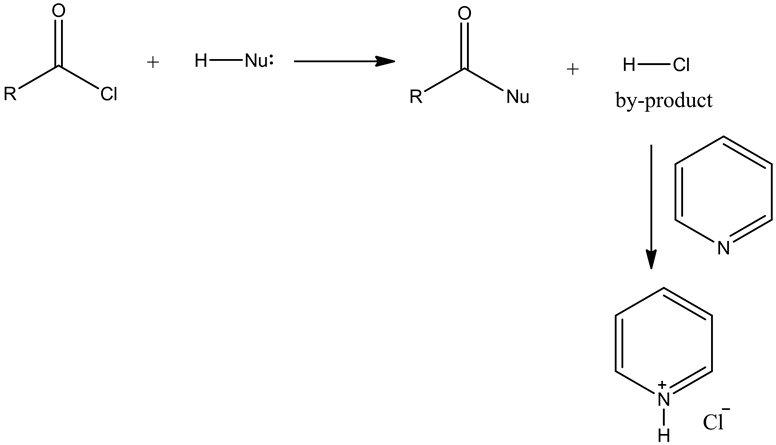
The concept used to solve this question is formation of amide using acyl chloride.
Acid chlorides reacts with primary amine and forms a secondary amide.
Fundamentals
Acyl chloride reacts with nucleophile and forms a substitution product with HCl as a by-product.
Answer:
The given amine is as follows:
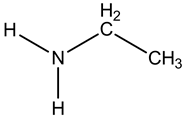
It is a primary amine.
Explanation:
The nitrogen is bonded with two hydrogen atoms and one ethyl group.
So, it is a primary amine.
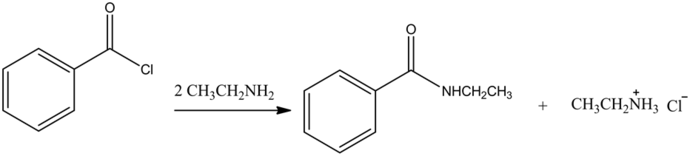
The reaction is as follows:
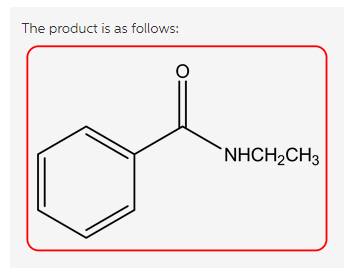
Explanation:
Treatment of benzoyl chloride with two equivalents of ethyl amine and forms N-ethylbenzamide and ethyl ammonium salt.
Here one equivalent of ethyl amine acts as a nucleophile to replace the -Cl group and forms the substitution product. The second equivalent of ethyl amine reacts as a base with the HCl by product and forms an ethyl ammonium salt.