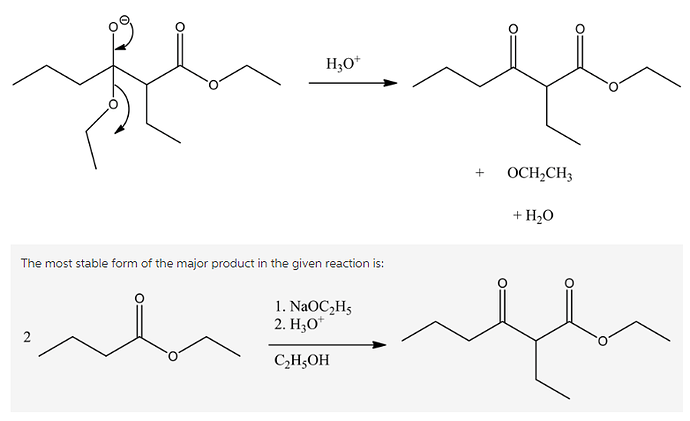
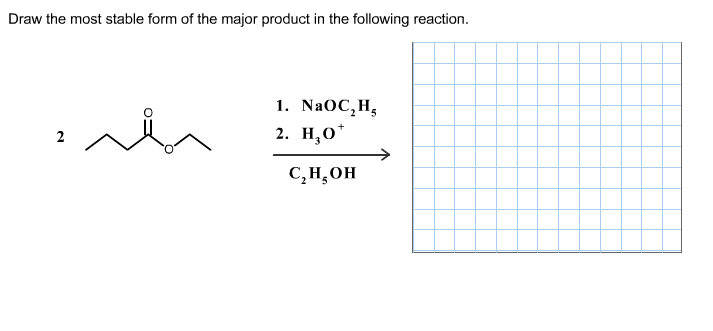Draw the most stable form of the major product in the following reaction.
Concepts and reason
Claisen Condensation:
The claisen condensation is similar to aldol condensation. An ester has alpha hydrogens, which undergo the condensation reaction.
Fundamentals
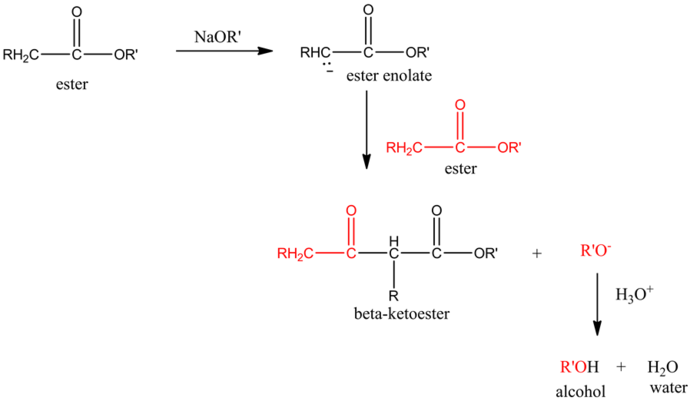
The base that is most commonly used in the claisen condensation reaction is alkoxide, ![]() .The ester, on reaction with base, forms an ester enolate, which again reacts with another molecule of ester.
.The ester, on reaction with base, forms an ester enolate, which again reacts with another molecule of ester.
The enolate is a good nucleophile, and ester carbonyl carbon is electrophilic by nature.
The final product of this reaction is a beta-ketoester.
The overall scheme of claisen condensation is as follows:
Answer:
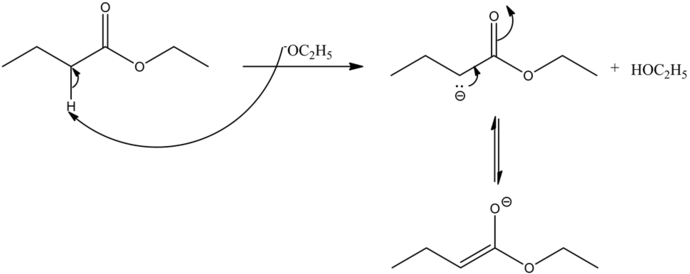
The base abstracts the alpha hydrogen of ester, which forms a carbanion. The carbanion donates its electrons to form a highly resonance-stabilized enolate, anion. The enolates are very strong nucleophiles.
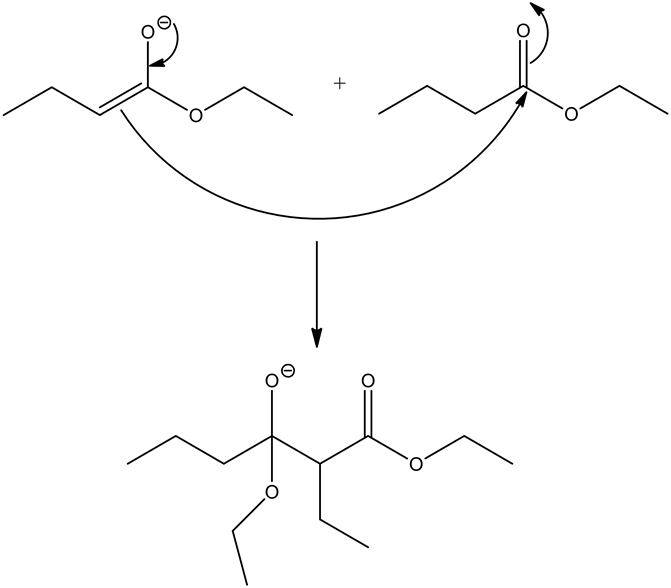
The enolates are very strong nucleophiles, and the carbon of ester carbonyls are very strong electrophiles by nature. Hence, the nucleophilic carbon from the ester enolate, attacks the electrophilic carbonyl carbon, which gives a condensed product.
The reaction of alkoxide with hydronium ion gives beta-ketoester with alcohol, and water molecules as side products. Ethanol is a solvent that is used in this reaction, which does not involve in the reaction mechanism.