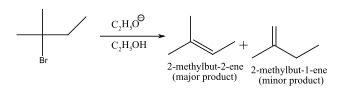
Draw the major organic product (other than ethanol) formed in the following reaction
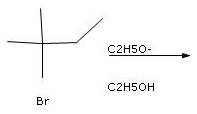
Concepts and reason
The problem is based on the concept of elimination reaction in alkyl halide. Mechanism of elimination can be ![]() depending upon the stability of carbocation.
depending upon the stability of carbocation.
The order of stability of carbocation is as follows:
Fundamentals
In elimination, final product is alkene which is formed following Saytzeff rule which states that more substituted alkene is more stable.
Answer:
Mechanism for the reaction will be E1 and formation of alkene take place by following Saytzeff rule.
Explanation:
In present case, alkyl halide is tertiary therefore, carbocation formation is favored. Mechanism can be ![]() depending on nature of nucleophile. Nucleophile is
depending on nature of nucleophile. Nucleophile is ![]() which is strong base therefore, E1 will be favored.
which is strong base therefore, E1 will be favored.
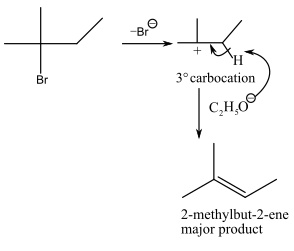
In first step, elimination of bromide ion takes place resulting formation of more stable tertiary carbocation. After the formation of carbocation in second step, base abstract hydrogen atom from carbon with less number of hydrogen atoms (Saytzeff rule) resulting formation of more substituted alkene that is 2-methylbut-2-ene.
The mechanism of the reaction will be as follows:
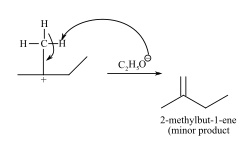
There could be another possibility but the product (2-methylbut-1ene) so formed is less substituted alkene therefore less stable or minor product.

Explanation:
In this reaction, ethoxide ion is a strong base therefore, elimination reaction take place which results in the formation of more substituted double bond according to Saytzeff rule, therefore, major product is as follows: