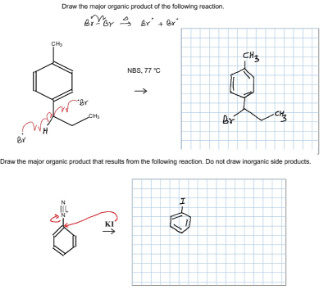
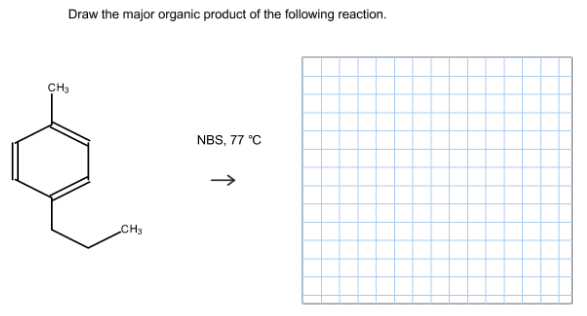
Draw the major organic product of the following reaction.
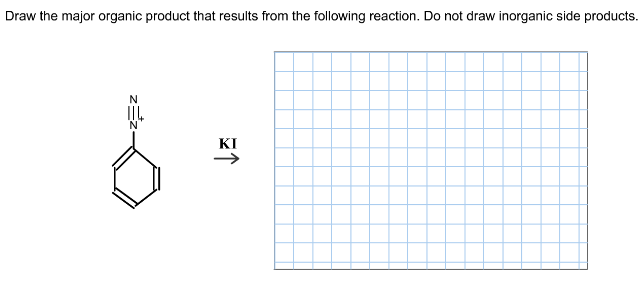
Answer:
(1)
NBS is used to add Br at allylic position means at the carbon next to the C=C bond. In the given compound we have two allylic positions. one in methyl and another in propyl. This reaction takes via free radical mechanism. Propyl gives 2 degree free radical where as methyl gives 1 degree free radical. we know that 2 degree free radicals more stable than 1 degree.
(2)
This is Sandmayer reaction in which substitution of amine is done by using a nucleophile. please note that amine is converted to diazonium salt. In this equation we are already given with diazonium salt. So, substitution would take place and the product would be as given in the picture…