Draw the Fischer projection for the monosaccharide drawn as a haworth projection below.
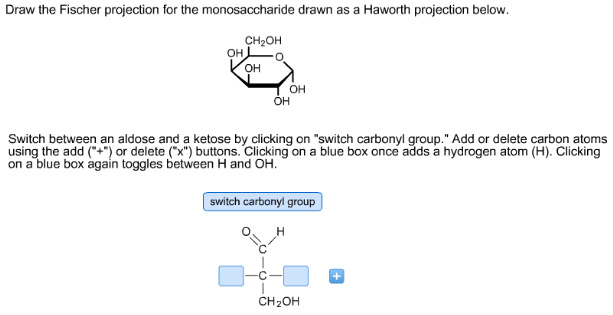
Concepts and reason
The concept used to solve this problem is converting of Haworth projection to Fischer projection. The cyclic structure is given in the question, so draw in straight chain. Place the –OH group at appropriate positions using the rules of converting cyclic ring structure to straight chain structure.
Fundamentals
In the Fischer projection, if the carboxyl group is on the top, R group is on the bottom of the vertical axis, and the amino group is on the right side then it is known as D-amino acid. Similarly, if the carboxyl group is on the top, R group is on the bottom of the vertical axis and the amino group is on the left side then it is known as L-amino acid.
The substituents/groups which are facing towards up, must be placed on left side. Similarly, the groups which are facing towards down, must be placed on right side.
Answer:
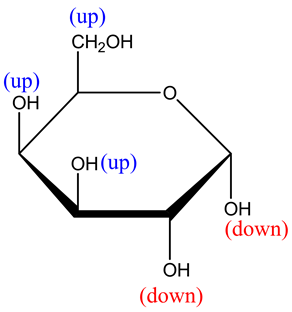
The structure is as follows:
Explanation:
In this structure, the two –OH groups are facing towards down, remaining two –OH and groups and one ![]() is facing towards up.
is facing towards up.
The Fischer projection is as follows:
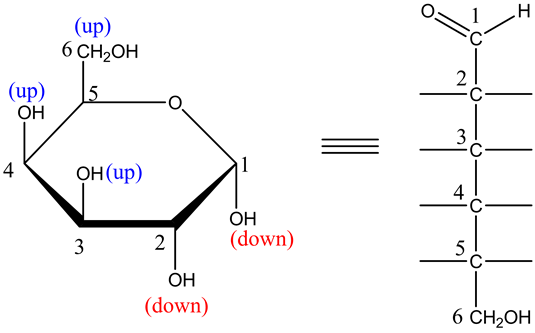
In this structure, draw the long chain carbon skeleton by placing the –CHO on top and ![]() on the bottom.
on the bottom.
The structure is as follows:
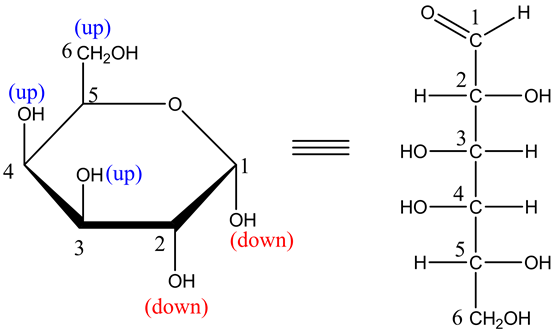
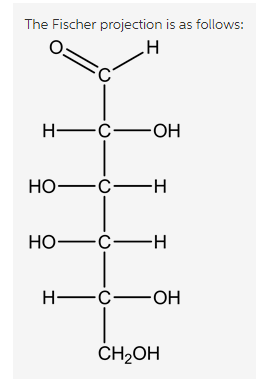
Explanation:
The two –OH groups that are facing towards up are placed on left side. Similarly, the –OH groups that are facing towards up are placed on right side. The anomeric carbon becomes C=O at C1.