Draw the ether formed when ethyl alcohol is heated in the presence of concentrated sulfuric acid.
Concepts and reason
In organic chemistry, ethers are a functional group that contains ( R - O - R) bonding, and ‘R’ is an alkyl group (Ex methyl, ethyl or aryl groups).
The oxygen atom is bonded to two alkyl groups. The ethers are non-polar by nature. The alcohol undergoes an acid-catalyzed condensation reaction to produce symmetrical ethers.
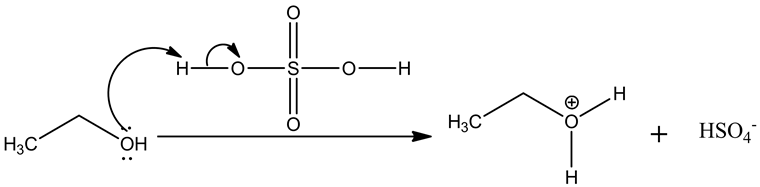
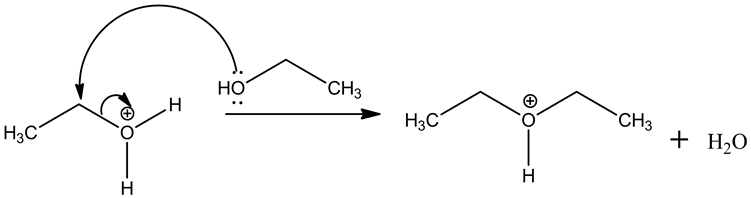
The nucleophilic oxygen of alcohol attacks the electrophilic carbon of alkyloxonium ion, leading to the removal of water molecule.
Fundamentals
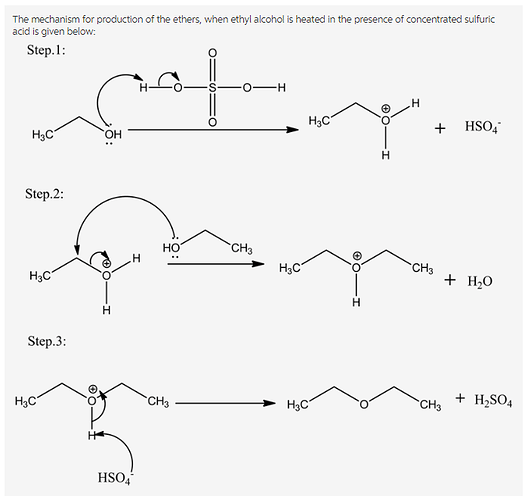
The synthesis of ethers takes place from alcohol under acid catalyst. The mechanism of the reaction mainly involves of three steps:
Step.1: Protonation of alcohols.
Step.2: Nucleophilic substitution ![]() .
.
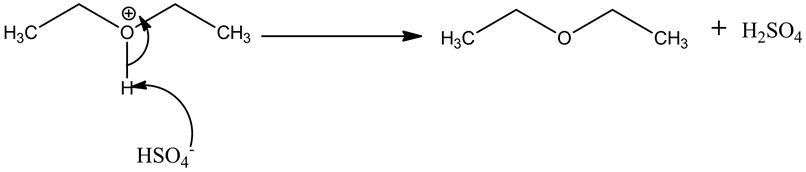
Step.3: Deprotonation to give symmetrical ethers.
Answer:
The mechanism of the reaction is given below:
The mechanism of the reaction is given below:
The mechanism of the reaction is given below: