Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose?
Answer:
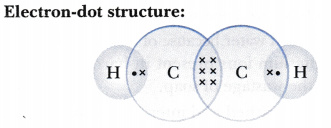
Ethyne burns in air with a sooty flame because of incomplete combustion caused by the limited supply of air. But when burnt at 3000°C in oxygen it gives a clean flame because of complete combustion. This oxy-acetylene flame is used for welding.
Such a high temperature cannot be achieved without mixing oxygen.
Therefore, a mixture of ethyne and air is not used for welding.