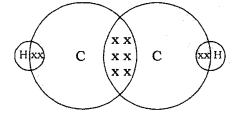
Draw the electron-dot structure for ethyne.’ A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose?
The electron-dot structure for ethyne, C2H2, is shown as:
When ethyne (acetylene) is burnt with oxygen, it gives a clean oxyacetylene flame with temperature of around 3,000
A °C because of complete combustion.
2C2H2 + 5O2 → 4CO2 + 2H2O + Heat
However, when ethyne is burnt with air, it gives a sooty flame. This is due to incomplete combustion caused by limited supply of oxygen obtained from air.
Thus, such a high temperature is not attained. Therefore, a mixture of ethyne and air is not used for welding purpose.